Range: Entire Indo-Pacific.
Description: Small to medium-sized, moderately light to solid. Last whorl usually broadly to broadly ventricosely conical or even ovate; outline faintly to pronouncedly convex; left side straight to concave at base. Aperture variably wider at base than at shoulder. Shoulder subangulate to angulate, tuberculate. Spire of low to moderate height, outline concave to convex. Larval shell multispiral. Postnuclear spire whorls tuberculate to strongly tuberculate. Teleoconch sutural ramps flat to concave, with 1-7 spiral grooves in later whorls; last ramp may have additional spiral striae. Last whorl variable in surface sculpture; largely smooth shells with well separated faint spiral ribs at base grade into shells with distinct granulose ribs from base to adapical third.
| Shell Morphometry | ||
|---|---|---|
| L | 20-47 mm | |
| RW | 0.08-0.45 g/mm | |
| RD | 0.68-0.84 | |
| PMD | 0.69-0.89 | |
| RSH | 0.10-0.23 | |
Ground colour pale grey, pale beige to pink or pale purple; often with several shades merging together. Last whorl with pale, occasionally obsolete spiral bands below shoulder and centre. Variously sized markings of brown, black or olive, spirally aligned on either side of subcentral band, either separate or fusing into 2 solid colour bands. Variably spaced spiral rows of alternating white and dark dots or dashes from base to shoulder; occasionally, with additional diagonal or zigzag-shaped opaque white markings. Larval whorls grey or light violet to red. Teleoconch spire radially maculated with varying brown to black blotches or bundles of fine lines. Aperture bluish to brownish grey, with pale bands below shoulder and centre.
Periostracum yellow to yellowish grey, thin, variously translucent, and smooth.
Colour pattern of animal (Pl. 74, Fig. 9) variable within local populations and between populations from different areas: Foot white to brown or brownish green (form aristophanes from Marshall Is.). Dorsum of foot sometimes spotted with purple or black, sometimes with orange or red anterior edge, and often a dotted black marginal line joining a large, brown or black, trilobate blotch anteriorly. Side of foot may be lighter iin colour posteriorly, with or without a reddish tinge. Sole of foot often edged with red anteriorly or at both ends, with or wothout purple and black mottling. Rostrum uniformly light to darker brown, pink, orange or brown tipped with orange, sometimes finely mottled with black. Tentacles beige with darker tips, brown or solid white. Siphon white to dark reddish brown, variously dotted with brown to black, usually with a variably wide red tip; black mottling either on entire siphon or only behind the reddish distal zone; occasionally with additional densely set white dots or heavy black streaks (Kohn, 1978a, unpubl. observ.; Lewis, 1979; Chaberman, pers. comm., 1981, Person, unpubl. observ.). In the N. Red Sea, animals rather uniformly coloured; scattered brown spots on a greyish white ground (Fainzilber et. al., 1992).
Radular teeth small and stout, with an adapical barb opposed to long blade; serration short, ending anterior to the central constriction of the shaft; base with a distinct spur (James, 1980; Bandel, 1984; Kohn, unpubl. observ.).
Habitat and Habits: Intertidal to about 10 m, at protected or exposed sites. In southern Africa, usually occurs in the middle part of the intertidal zone, living in or on sand pockets, sand- filled crevices and in sand along edges of rocks as well as among sea- weeds and oysters; reported to favour semi-sheltered or sheltered habitats (Kilburn & Rippey, 1982; Grosch, pers. comm., 1989). In the Red Sea, it lives on the inner reef flat among rocks and boulders (Sharabati, 1984). In India, intertidally on rough limestone benches and in or on rubble or coarse sand beneath or near coral rocks (Kohn, 1978a); in Sri Lanka, both intertidally and slightly subtidally on reef flats inhabiting the same microhabitats as in India and additionally bare coral rocks with or without algal turf (Kohn, 1960). On intertidal reefs in Thailand and Indonesia, in sand-filled depressions, large areas of sand, and on bare limestone pavement (Kohn & Nybakken, 1975). Data from intertidal reef rock benches of the Seychelles, E. Australia and Micronesia indicate a similar selection of microhabitats as in the areas mentioned above (Leviten & Kohn, 1980). In New Caledonia, coral between 1-3 m is the main microhabitat (Tirard, pers. comm., 1989). In the Maldive and Chagos Archipelagoes, it occurs on intertidal limestone benches as well as on subtidal reef flats (Kohn, 1968b; Leviten & Kohn, 1980). According to Lewis (1979), the typical form of C. coronatus in Fiji lives on moderately coarse sand near the edge of the reef; form aristophanes (see below) usually occurs farther inshore on finer sand and mudflats. The two forms overlap only slightly in habitat. Findings from the Philippines indicate a similar conchological and ecological separation. C. coronatus feeds mainly on errant polychaetes (Eunicidae, Glyceridae, Nereidae), rarely consuming sedentary species (Capitellidae). Egg capsules are deposited singly or in short rows of 3 - 10 on the underside of coral or limestone rocks. Collective clusters may be made by several females. Capsules more or less quadrangular with a short stalk and a small basal plate, measuring 4.5-12.5 x 4.0-11.0 mm. Number of capsules per capsule mass is 14-83, number of eggs per capsule mass 25,000-52,000 and number of eggs per capsule 330-3,000. Egg diameter of 150-180 ┬Ám predicts a minimum pelagic period of about 28-25 days (Kohn, 1961b; Perron & Kohn, 1985).
Discussion: C. coronatus resembles the typical form of C. m. miliaris in shell characters and often also in the colouration of the animal. Typical C. m. miliaris can be distinguished by its generally less ventricose last whorl, consistently angulate shoulder with generally more prominent tubercles and in the presence of a central pad as well as an abapical ridge within the aperture. C. miliaris lacks the variously sized brown or olive markings on the last whorl, the spiral rows of dots and dashes finer, and white instead of dark markings are the dominant pattern element. In addition, the aperture of C. m. miliaris is paler brownish, pinkish and violet, rather than blue, grey and darker brown). Where both species occur sympatrically, differences can usually be observed in the distribution pattern across the habitat, the diet composition and the microhabitats chosen. Some specimend of C. coronatus sre similar to C. abbreviatus in shell shape and colouration but differ in the colour of animals and apertures (bluesh to brownish grey vs. brownish violet); the intermittent white markings are absent from the dotted spiral lines on the last whorl of C. abbreviatus. In Tahiti, Fiji and Philippines, typical C. coronatus and a form corresponding with C. aristophanes as redescribed by Cernohorsky (1964), differ in a number of shell caracters as well as in habitat. Form aristophanes (Pl. 7, Figs. 29-31) has a narrower, less ventricose last whorl with a straighter outline, and fewer spiral grooves on the later sutural ramps. It more often has a bluish or greyish last whorl with more pronounced pale spiral bands. In other regions (e.g. Solomon Is., Japan, Maldives, Oman and Zanzibar), the two forms intergrade. In the type locality of C. aristophanes, "Philippines Is.," they are separable. Based on data from such areas, Cernohorsky (1964) and Lewis (1979) considered C. aristophanes a valid species. However, data from the entire range favour ranking C. aristophanesas as a form of C. coronatus. Coomans et al. (1981) reached the same conclusion.
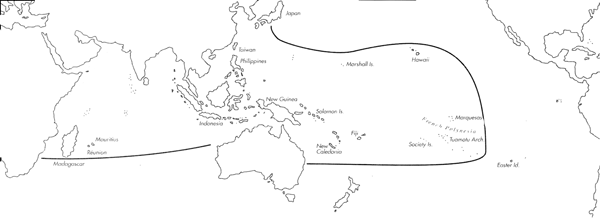
C. coronatus Range Map
This section contains verbatim reproductions of the accounts of 316 species of Conus from the Indo-Pacific region, from Manual of the Living Conidae, by R÷ckel, Korn and Kohn (1995). They are reproduced with the kind permission of the present publisher, Conchbooks.
All plates and figures referred to in the text are also in R÷ckel, Korn & Kohn, 1995. Manual of the Living Conidae Vol. 1: Indo-Pacific Region.
The range maps have been modified so that each species account has it own map, rather than one map that showed the ranges of several species in the original work. This was necessary because each species account is on a separate page on the website and not confined to the order of accounts in the book.